Morning After Pill Won't Work for Women Heavier Than 176lbs; Might Not Work for Those Weighing More Than 165lbs

The U.S. Food and Drug Administration (FDA) is now considering whether or not to start requiring local makers of emergency contraceptive pills to add weight warnings to product labels after a French maker of a pill similar to Plan B revealed that the drug doesn't work at all for women heavier that 176lbs, and begins to lose effectiveness for those weighing more than 165lbs.
HRA Pharma, the French manufacturer of the emergency contraceptive Norlevo, is currently changing its packaging to warn women of the weight limits after European pharmaceutical regulators approved amendments to the labels on Nov. 10 based on scientific data highlighting the weight problem, according to a report in Mother Jones.
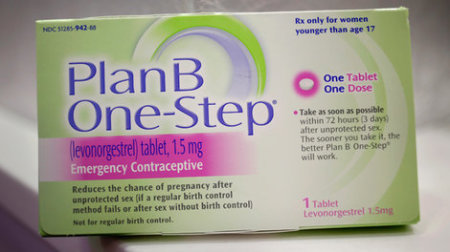
And with the average American woman weighing 166lbs, according to the CDC, this could spell trouble for American women using popular emergency contraceptive pills like 'Plan B One Step', 'Next Choice One Dose', 'My Way' and other generic brands with doses of the drug containing a similar chemical makeup to Norlevo.
These pills, which depend on an ingredient called levonorgestrel to prevent pregnancies, are reportedly the most effective emergency contraceptives available over the counter.
While no such warning has yet been issued in the U.S., the FDA says it is looking into whether or not they will take action based on the new findings.
"The FDA is currently reviewing the available and related scientific information on this issue, including the publication upon which the Norlevo labeling change was based," FDA spokeswoman Erica Jefferson told Mother Jones in an email. "The agency will then determine what, if any, labeling changes to approved emergency contraceptives are warranted."
While she declined to say when the FDA began their review of the information, Jefferson highlighted that both brand and generic drug makers are obligated to ensure that their labeling is accurate.
"When new information becomes available that causes information in drug product labeling to be inaccurate, both brand and generic drug application holders must take steps to change the content of their product labeling," she said.
Generic drug makers are required by the FDA to reflect the same product information that appears on brand name drugs. Generic drug makers, like "My Way" and "Next Choice," however, cannot make updates to brand name product information like "Plan B One-Step" manufactured by Teva Pharmaceutical Industries. Only the FDA or Teva Pharmaceutical Industries can make product information changes to reflect the new research.
Meanwhile, Anna Glasier, professor of obstetrics and gynecology at the University of Edinburgh who wrote the study that triggered HRA Pharma's review, told CNN that heavier women could still use the contraceptive pill.
"You are probably better to take [levonorgestrel emergency contraceptives] after unprotected sex than just to leave it to chance even if you are obese," she said.





















